¬ü±Ð±Âµo©úª`®g¶K¥¬ ¥´¬Ì]¤£¥²¦A®Á°w
2017-06-29
1396
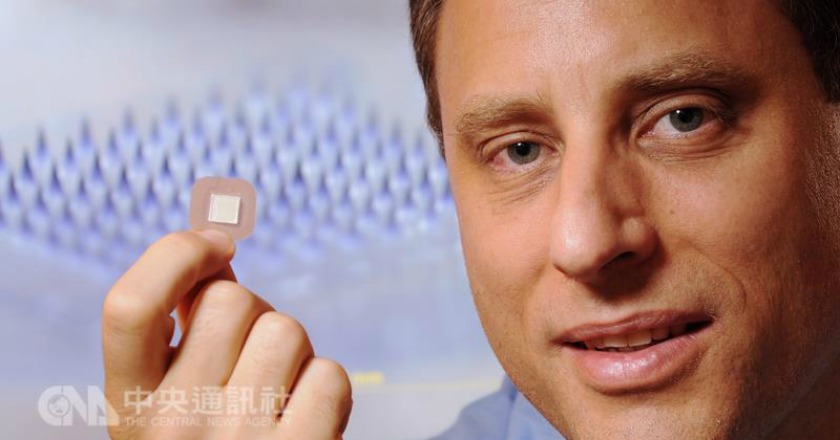
°wÀY¬ï¹L¦×Å骺Àþ¶¡¯u¥O¤H®£Äß¡A¬ü°ê³ìªv¨È²z¤u¾Ç°|¥Í¤Æ¤uµ{±Ð±Â´¶³Ò´µ¥§¯÷¡Aµo©ú¦pÅ]°ÀÖªºª`®g¶K¥¬¡A¥iÀø¡«UºÙ·w°wªº°wÀY®£Ä߯g¡C
¤¤¥¡ªÀ³ø¾É¡A°wÀY¬ï¹L¦×Å骺Àþ¶¡¯u¥O¤H®£Äß¡A¬ü°ê³ìªv¨È²z¤u¾Ç°|¥Í¤Æ¤uµ{±Ð±Â´¶³Ò´µ¥§¯÷¡Aµo©ú¦pÅ]°ÀÖªºª`®g¶K¥¬¡A¥iÀø¡«UºÙ·w°wªº°wÀY®£Ä߯g¡C
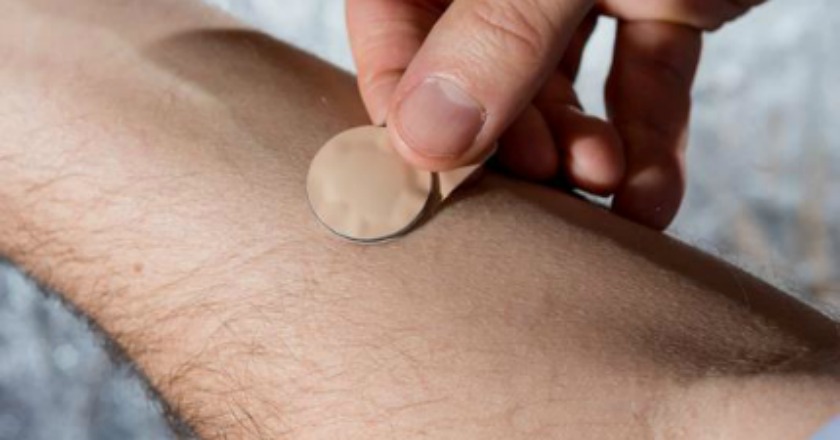
¡u¬¥§üÁF®É³ø¡v¡]LA Times¡^³ø¾É¡A´¶³Ò´µ¥§¯÷¡]Mark Prausnitz¡^ªºª`®g¶K¥¬¡Aªí±ªþµÛ¶W¹L100Ó¤£¨ì1¤½Íùªº³S¬Ã°wÀYµS¦pÅ]°ÀÖ¡A·íª`®g¶K¥¬µLµh±K¦X©ó¤HÅ骺¥Ö½§¡A¶K¥¬¸Ìªº¾¯¶q¤]½w½wª`¤JÅ餺¡C
¤j¬ù20¤ÀÄÁ«á¡A¦ÛӨ༹±¼¥¦´Nºâ§¹¦¨ª`®g¡A«D±`²«K¡A¥B¯f±w§¹¥þ¬Ý¤£¨ì°wÀY¤£¦Ü´ý¨¤£¦Û¦b¡AÂåÅ@¤Hû¤]µL»Ý»P¬Ý¨ì°wÀY´Nú¾x¤£¤îªº¤pªB¤Í©Ô§è¡A¯u¬O¤@Á|¼Æ±o¡A¦³§UÂå¯fÃö«Y¡C
¤×¨ä¨C³{¬y·P¸vh®É¸`¡AºÉºÞ¬ü°ê¦U¯Å½Ã¥ÍÂåÀø³æ¦ì¡AÀWÀW©IÆ~ª`®g¬y·P¬Ì]ªº«n©Ê¡A¤£¹LÄ@·N¬°¤F¨Åé°·±d½t¬G¨ì¶E©Òªá¿ú®Á°wÀYªº¦¨¤H¡A¤ñ¨Ò§C©ó4¦¨¡A¦ôp¬ü°ê¨C¦~¦]¬y·PP¦ºªº¯f±w¤¶©ó1¸U2000¦Ü5¸U6000¤H¤£µ¥¡C
¦]¦¹¬ü°êÂå¾Ç¬É¬Ý¦n¡A´¶³Ò´µ¥§¯÷ªºµo©ú¥i¹B¥Î©ó¬y·P¬Ì]ªºª`®g¡A¦]¹êÅçÃÒ©ú¡A¹B¥Îª`®g¶K¥¬»P°wÀYª`®g¬y·P¬Ì]¡A¦b¤HÅ餺¥Í¦¨ªº§ÜÅé¿@«×¥i»¡¬OÁÍ©ó¤@P¡C
·íµM¤£¥u¬O¬y·P¬Ì]¡A¨ä¥L¹³¬O³Â¯l¬Ì]µ¥¡A¤é«á¤]¯à±Ä¥Î³o´ÚµLµhª`®gªºª`®g¶K¥¬¡A¥i¦³®Ä¤Æ¸Ñ«UºÙ·w°wªº°wÀY®£Ä߯g¡]needle-phobia¡^¡C1060629
¥Zµn¦bÂå¾Ç´Á¥Z¡u¨ë¯Þ°w¡v¡]The Lancet¡^ªº¬ã¨s¡GThe safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial
¡i¹Ï¤ù¨Ó·½¡G¤¤¥¡ªÀ¡]ºK¦Û¬ü°ê³ìªv¨È²z¤u¾Ç°|ºô¯¸¡^¡j
¡u¬¥§üÁF®É³ø¡v¡]LA Times¡^³ø¾É¡A´¶³Ò´µ¥§¯÷¡]Mark Prausnitz¡^ªºª`®g¶K¥¬¡Aªí±ªþµÛ¶W¹L100Ó¤£¨ì1¤½Íùªº³S¬Ã°wÀYµS¦pÅ]°ÀÖ¡A·íª`®g¶K¥¬µLµh±K¦X©ó¤HÅ骺¥Ö½§¡A¶K¥¬¸Ìªº¾¯¶q¤]½w½wª`¤JÅ餺¡C
¤j¬ù20¤ÀÄÁ«á¡A¦ÛӨ༹±¼¥¦´Nºâ§¹¦¨ª`®g¡A«D±`²«K¡A¥B¯f±w§¹¥þ¬Ý¤£¨ì°wÀY¤£¦Ü´ý¨¤£¦Û¦b¡AÂåÅ@¤Hû¤]µL»Ý»P¬Ý¨ì°wÀY´Nú¾x¤£¤îªº¤pªB¤Í©Ô§è¡A¯u¬O¤@Á|¼Æ±o¡A¦³§UÂå¯fÃö«Y¡C
¤×¨ä¨C³{¬y·P¸vh®É¸`¡AºÉºÞ¬ü°ê¦U¯Å½Ã¥ÍÂåÀø³æ¦ì¡AÀWÀW©IÆ~ª`®g¬y·P¬Ì]ªº«n©Ê¡A¤£¹LÄ@·N¬°¤F¨Åé°·±d½t¬G¨ì¶E©Òªá¿ú®Á°wÀYªº¦¨¤H¡A¤ñ¨Ò§C©ó4¦¨¡A¦ôp¬ü°ê¨C¦~¦]¬y·PP¦ºªº¯f±w¤¶©ó1¸U2000¦Ü5¸U6000¤H¤£µ¥¡C
¦]¦¹¬ü°êÂå¾Ç¬É¬Ý¦n¡A´¶³Ò´µ¥§¯÷ªºµo©ú¥i¹B¥Î©ó¬y·P¬Ì]ªºª`®g¡A¦]¹êÅçÃÒ©ú¡A¹B¥Îª`®g¶K¥¬»P°wÀYª`®g¬y·P¬Ì]¡A¦b¤HÅ餺¥Í¦¨ªº§ÜÅé¿@«×¥i»¡¬OÁÍ©ó¤@P¡C
·íµM¤£¥u¬O¬y·P¬Ì]¡A¨ä¥L¹³¬O³Â¯l¬Ì]µ¥¡A¤é«á¤]¯à±Ä¥Î³o´ÚµLµhª`®gªºª`®g¶K¥¬¡A¥i¦³®Ä¤Æ¸Ñ«UºÙ·w°wªº°wÀY®£Ä߯g¡]needle-phobia¡^¡C1060629
¥Zµn¦bÂå¾Ç´Á¥Z¡u¨ë¯Þ°w¡v¡]The Lancet¡^ªº¬ã¨s¡GThe safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial
¡i¹Ï¤ù¨Ó·½¡G¤¤¥¡ªÀ¡]ºK¦Û¬ü°ê³ìªv¨È²z¤u¾Ç°|ºô¯¸¡^¡j
§@ªÌ¤¶²Ð
©µ¦ù¾\Ū



![¥_³¡1¤ë¤jÀ¦¬V¦Ê¤é«y¤µ¦~¥»¤gº¨Ò ¯eºÞ¸p´£¿ô¥¥°ü¥´¬Ì]](/km/sendbinary_Mall.asp?path=C:\WebSite\Formosa\News\upload\20240319000108Mg.jpg)
![65·³¥H¤Wµ¥°ª·ÀI±Ú¸s ²Ä2¾¯XBB¬Ì]¶}¥´](/km/sendbinary_Mall.asp?path=C:\WebSite\Formosa\News\upload\20240409000087g.jpg)
